About Fuel Cells
It is well known that water is broken down into hydrogen and oxygen through electrolysis.
Conversely, fuel cells are power-generating devices that operate by directly taking out electricity through the electrochemical reaction of hydrogen and oxygen, from air, making water and completing the cycle.
The first invention of fuel cells was a development by Sir William Robert Grove in 1839. At right, the illustration shows inverted test tubes standing in sulfuric acid electrolyte solution, into which were placed platinum black-covered platinum electrodes. Then, electrolysis was applied to generate hydrogen and oxygen gases. Later on, the fuel cells were connected to a voltmeter, which provided strong evidence that electric voltage was generated when the electrode containing hydrogen became negative. It was then discovered that water could be decomposed by electrical energy when these fuel cells were connected in series.
Since fuel cells do not use heat during the energy conversion process, unlike other electricity generating methods, fuel cells provide the most efficient form of energy generation.
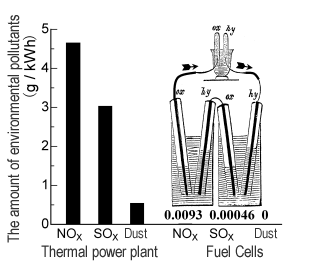
Hydrogen-based energy does not release any environmental pollutants. When applying hydrogen-rich gas after reforming natural gas, it also greatly reduces the amounts of emissions, as shown at right.
If you use 1 kWh (continuous electrical power for household use for one hour), for example, 4.6 g of NOx and 3 g of Sox would be released into the air. (We need to be aware of energy conservation and environmental pollutants!) However, in the case of applying fuel cells, NOx can be reduced by a factor of five hundred, and SOx by a factor of ten thousand. Because fuel cells do not directly burn fuels, there is no possibility of particulates being produced as asthma- / or lung cancer-causing culprits. Other advantages of using fuel cells are that the higher energy efficiency reduces fuel consumption, and that the amount of carbon dioxide emissions, a contributing factor to global warming, can be reduced by up to half.
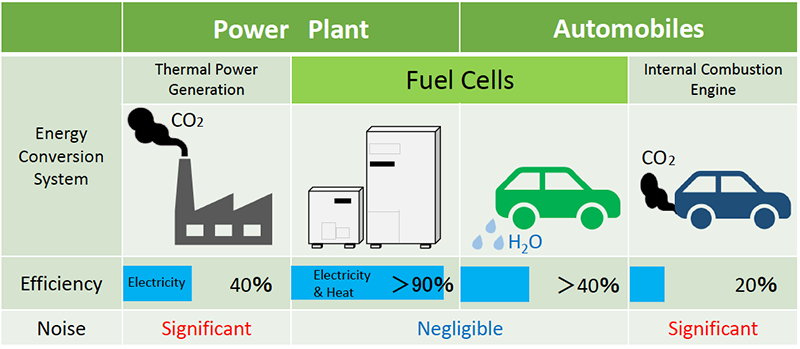
Fuel cells are not only used for power plants for household purposes, but also for electrical vehicle (EV) power supplies. Although battery-powered vehicles require the battery to be charged after a certain distance, fuel cell-based EVs only need refueling and can run continuously. If fuel cells were applied to EVs, the total energy conversion efficiency would be approximately 2.5 times that of gasoline-fueled vehicles. This clearly shows that fuel cells provide pure, clean energy, which contributes to energy conservation, as you can see in the figure below.
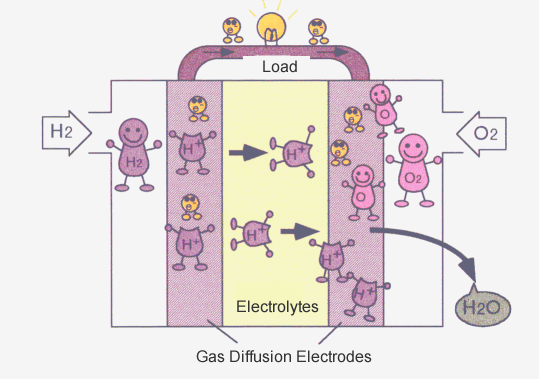
Fuel cells are composed of electrolytes (liquid or solid) and two gas diffusion electrodes. The electrode function is to break down the reactive gases into ions and electrons (the reverse reaction is also possible), and then the electrolytes allow the generated ions to pass through.
The categories of fuel cells are as follows: Alkaline Fuel Cells (AFC), Phosphoric Acid Fuels Cell (PAFC), Molten Carbonate Fuel Cells (MCFC), Solid Oxide Fuel Cells (SOFC) and Polymer Electrolyte Fuel Cells (PEFC), the latter being expected to be used in the future to supply power to electric vehicles.
Our laboratory has been dealing with research in these study areas for many years, covering a broad range of studies, including design and performance evaluation of fuel cell materials (electrocatalysts and ion conductive membranes). Our published study results have been widely recognized. Our international exchange research has also been flourishing.
Recently, we also conduct research on polymer electrolyte water electrolyzers.


